Introduction
The stoichiometry of carbon (C), nitrogen (N), and phosphorus (P) concentrations in plant organs reflects nutrient uptake, nutrient use efficiency, and environmental adaptation (Sardans et al., 2011; Schreeg et al., 2014; Li et al., 2017). Carbon forms the structural basis and substrate for plant metabolism (Thomas and Martin, 2012; Ma et al., 2018), N is a component of chlorophyll for photosynthesis, and P is a constituent of adenosine phosphate. Thus, C, N, and P stoichiometry can contribute to understanding nutrient compositions in the organs of different plant species in forest ecosystems (Sardans et al., 2011; Li et al., 2017).
The stoichiometry of nutrient ratios (C:N, C:P, and N:P) in plant organs is a critical indicator to characterize the changes in N and P allocation patterns and N- and P-use efficiencies during plant growth processes. However, the C:N:P stoichiometry in plant organs can be affected by abiotic (temperature, elevation, precipitation, and drought) and biotic (species composition, life form, and genotype) factors (Sardans et al., 2011; Li et al., 2017; Tang et al., 2018; Xing et al., 2022).
Understanding C, N, and P concentrations and stoichiometry of plant organs of different plant species is one of the most important aspects of forest nutrient management. For example, plant N:P ratios are widely used as indices of nutrient availability and limitation (Mo et al., 2015). The N:P ratios of stem and roots are more responsive indicators of soil nutrient availability than those of leaves (Schreeg et al., 2014). The nutrient concentration and stoichiometry pattern across various plant organs and forest types can reflect the trade-off of plant growth strategies (Xing et al., 2022). However, there is little information on the patterns of C:N, C:P, and N:P stoichiometry in the plant organs of different forests in Korean forest ecosystems.
Coniferous and broad-leaved forests in the warm-temperate forest zone of Korea are significant biomass resources because their carbon accumulation rates are comparable with other temperate forests (Son et al., 2014; Kim et al., 2020; Baek et al., 2021). Therefore, evaluating the nutrient concentrations and ratios of different plant organs according to forests is important to characterize nutrient uptake characteristics. This study aimed to assess the nutrient concentrations and stoichiometries of various plant organs (stem, bark, branches, and foliage) of the plant species of the warm temperate forest zone in Korean forests.
Materials and Methods
The study sites were located in one region (Namhe-gun) for Crypromeria japonica D.Don (CJ) and Quercus serrata Thunb. (QS) stands, three regions (Goseong-gun, Seogwipo-si, and Jindo-gun) for evergreen broadleaved stands (EB: Machilus thunbergii Siebold & Zucc., Neolitsea sericea (Blume) Koide., and Quercus glauca Thunb.), and two regions (Damyang-gun and Jinju-si) for Bamboo spp. stands (BB: Phyllostachys pubescens Mazel ex Lehaie, Phyllostachys bambusoides Siebold & Zucc., and Phyllostachys nigra var. henonis (Bean) Stapf ex Rendle.) in southern Korea. The mean annual precipitation and temperature for 30 years in the study areas are 1,518 mm year−1 and 13.4 °C for Jinju-si, 1,921 mm year−1 and 14.3 °C for Namhe-gun, 1,989 mm year−1 and 16.9 °C for Seogwipo-si, 1,470 mm year−1 and 14.2 °C for Goseong-gun, and 1,344 mm year−1 and 13.2°C for Damyamg-gun, respectively (Korea Meteorological Administration, 2022). The soils are well-drained, slightly wet or dry, brown forest soils originating from granite or granite gneiss with a sandy loamy or loamy texture for Namhae-gun, Jindo-gun, and Damyang-gun (Inceptisols of the USDA soil taxonomy). The soils are slightly dry, dark reddish-brown forest soils (Inceptisols of the USDA soil taxonomy) originating from sandstone or shale in Goseong-gun and Jinju-si and volcanic ash soils (Andosols of the USDA soil taxonomy) in Seogwipo-si. Additional information regarding the study site was given for the BB (Kim et al., 2018; Kwak et al., 2021), for the CJ and QS (Baek et al., 2021), and for the EB (Kim et al., 2019).
The experimental design consisted of three 20×20 m or 20×10 m plots at each site. Five diameter classes based on the DBH ranges were established for each site, and sample trees were randomly selected from each DBH class. In total, 10 trees for CJ, 10 trees for QS, 46 EB (10 trees for M. thunbergii, 10 trees for N. sericea, and 26 trees for Q. glauca), and 72 BB (26 for P. pubescens, 20 for P. bambusoides, and 26 for P. nigra var. henonis) were destructively sampled to measure the nutrient concentrations in the plant organs (Table 1). Felled samples were separated into plant organs (foliage, branches, bark, and stems) and each plant organ was weighed with electrical balance. Subsamples were obtained from each plant organ and oven-dried at 85°C for one week. All the investigations were performed in accordance with the technical standards of biomass measurement formulated by the Korea Forest Research Institute (2010). Detailed information regarding the sampled trees can be found elsewhere (Kim et al., 2018; Kim et al., 2019; Baek et al., 2021; Kwak et al., 2021). The dried samples were ground in a Wiley mill and passed through a 40-mesh stainless steel sieve. The C and N concentrations of the ground materials were determined using an elemental analyzer (Flash 2000; Thermo Scientific, Milan, Italy). The P concentrations were determined by dry ashing 0.5 g of the ground material at 470°C for 4 hours, digesting the ash with 3 mL of concentrated 5 M HCl, diluting the digest with 0.25 mL of concentrated HNO3 and 3 mL of concentrated 5 M HCl (Kalra and Maynard, 1991), and measuring the concentrations using ICP-OES (Optima 5300DV; Perkin Elmer, Shelton CT, USA).
Mean±standard error (min-max).
All data including nutrient concentration and stoichiometry of plant organs were analyzed using the general linear model procedure in SAS (SAS Institute, 2003) to determine the significant difference among the forests or plant organs at P < 0.05. Tukey’s multiple comparison test (P < 0.05) was used to compare the forests or plant organ means. Principal component analysis and correlations to analyze the associations of variables in the nutrients of plant organs were conducted using Canoco 5.1 (ter Braak and Šmilauer, 2018).
Results and Discussion
The mean C concentration of the plant species was significantly different among forests or plant organs (Figure 1). The mean C concentration in the stem was significantly higher in CJ (488 mg C g−1) than in QS (462 mg C g−1), BB (456 mg C g−1), or EB (453 mg C g−1), whereas the C concentration in the foliage was the lowest in BB (431 mg C g−1). However, the C concentration in the branches was not affected by forests. The highest C concentration in the stem of CJ could be due to the difference in lignin concentration determined by the genetic factors between tree species (Thomas and Martin, 2012; Ma et al., 2018). Chong and Park (2008) and Yang et al. (2017) reported that the lignin concentration of the stem was 320 mg g−1 for CJ, 223 mg g−1 for QS, 237 mg g−1 for EB, and 120-210 mg g−1 for BB, respectively. Similarly, Ma et al. (2018) observed that the C concentration of the plant organs is positively correlated (r = 0.54, P < 0.01) with the lignin concentration. In contrast to the stem, the lowest C concentration in the foliage of BB could be due to the highest N and P concentrations of the foliage (Figure 1). Carbon concentration in plant organ was negatively correlated with the nutrient concentration (Tang et al., 2018).
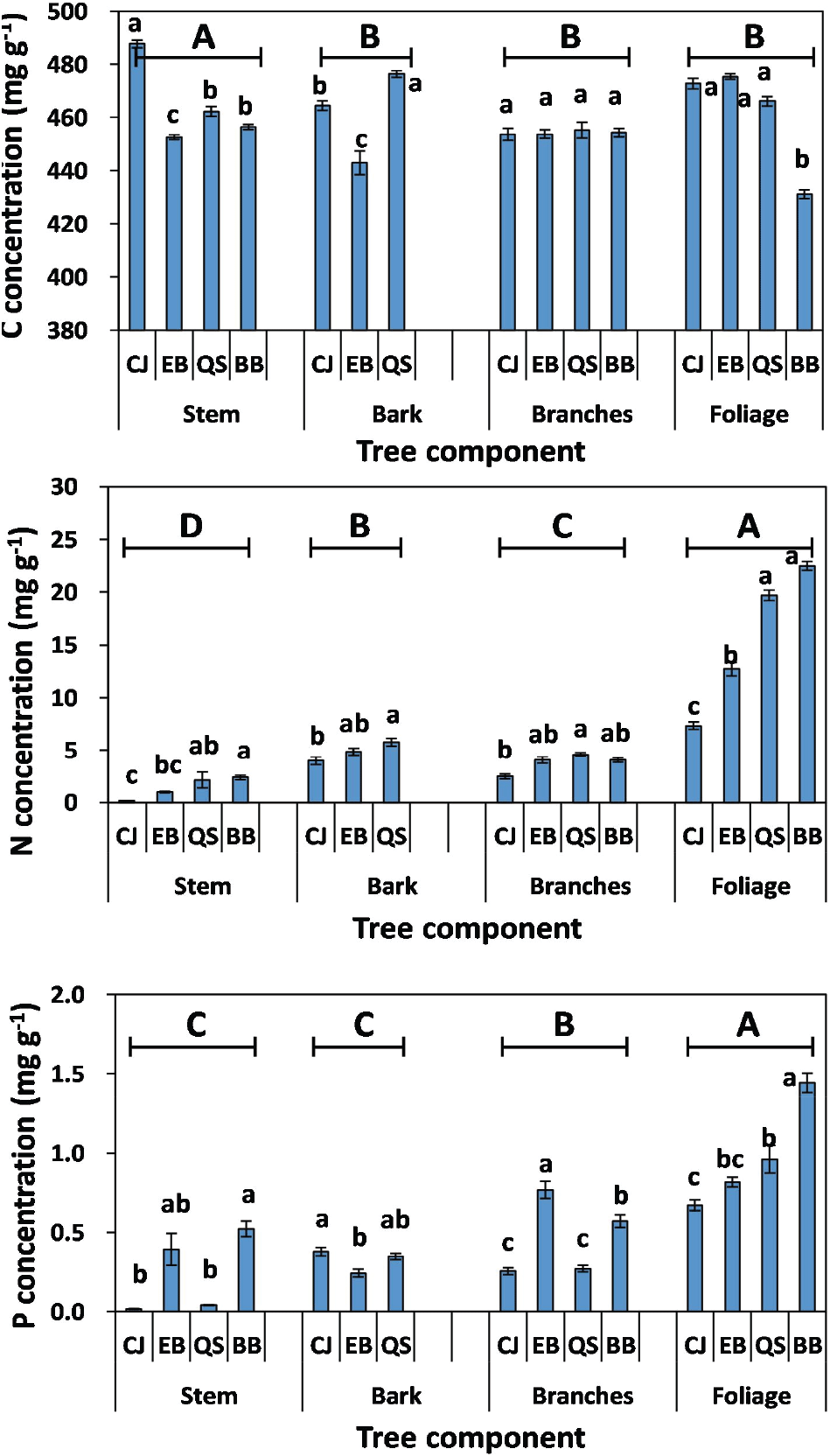
The mean N concentration in the foliage was significantly higher in BB (22.5 mg N g−1) or QS (19.7 mg N g−1) than in EB (12.7 mg N g−1) or CJ (7.3 mg N g−1). Similarly, the mean P concentration in the foliage was higher in BB (1.4 mg P g−1) than in QS (1.0 mg P g−1), EB (0.8 mg P g−1) or CJ (0.7 mg P g−1). The mean N concentrations in other plant organs, such as branches, bark, and stem, were significantly lower in CJ than in BB or QS. In contrast, the mean P concentration of branches, bark, or stem was not significantly different between CJ and QS. The N and P concentrations in plant organs showed different patterns among the forests, suggesting different demands for nutrients in plant organs in diverse forests. Fast plant metabolism in BB may require high N and P, resulting in N and P enrichment in the plant organs (Sardans et al., 2011). In contrast to BB, the lowest N and P concentrations of foliage and stem in CJ could be due to the intrinsic genetic factors of coniferous trees, and the dilution effect caused by high DBH compared with other forests (Table 1).
Mean C concentration in plant organs was significantly higher in stems (458 mg C g−1) than in other plant organs, such as bark (452 mg C g−1), branches (454 mg C g−1), and foliage (451 mg C g−1). However, N and P concentrations were the highest in the foliage (15.56 mg N g−1; 0.97 mg P g−1) and lowest in the stems (1.43 mg N g−1; 0.24 mg P g−1). The high N and P concentrations in the foliage indicate that the allocation of nutritional elements to the foliage was higher than those to the woody organs. Tang et al. (2018) suggest that foliage is the most important photosynthetic organ and requires high nutrient concentrations to improve its photosynthetic and metabolic capacities. In addition, Li et al. (2017) indicate that much variability in plant nutrient concentration has been accounted for by organ-specific differences and site-specific differences.
The mean C:N ratio in the stem, bark, and branches was significantly higher in CJ than in the other forests (Figure 2). The highest C:N ratio in each plant organ was expected in the CJ because of the lowest N concentration in each plant organ. Generally, the lowest C:N ratios were observed in the foliage, and the highest observed in the stem, which may be related to the transfer of carbohydrates from photosynthetic to structural organs. Previous study has indicated that an increase in the proportion of stems may lead to increased C:N ratios in the structural organs of plants, resulting in a high C:N ratio in the stems (He et al., 2015).
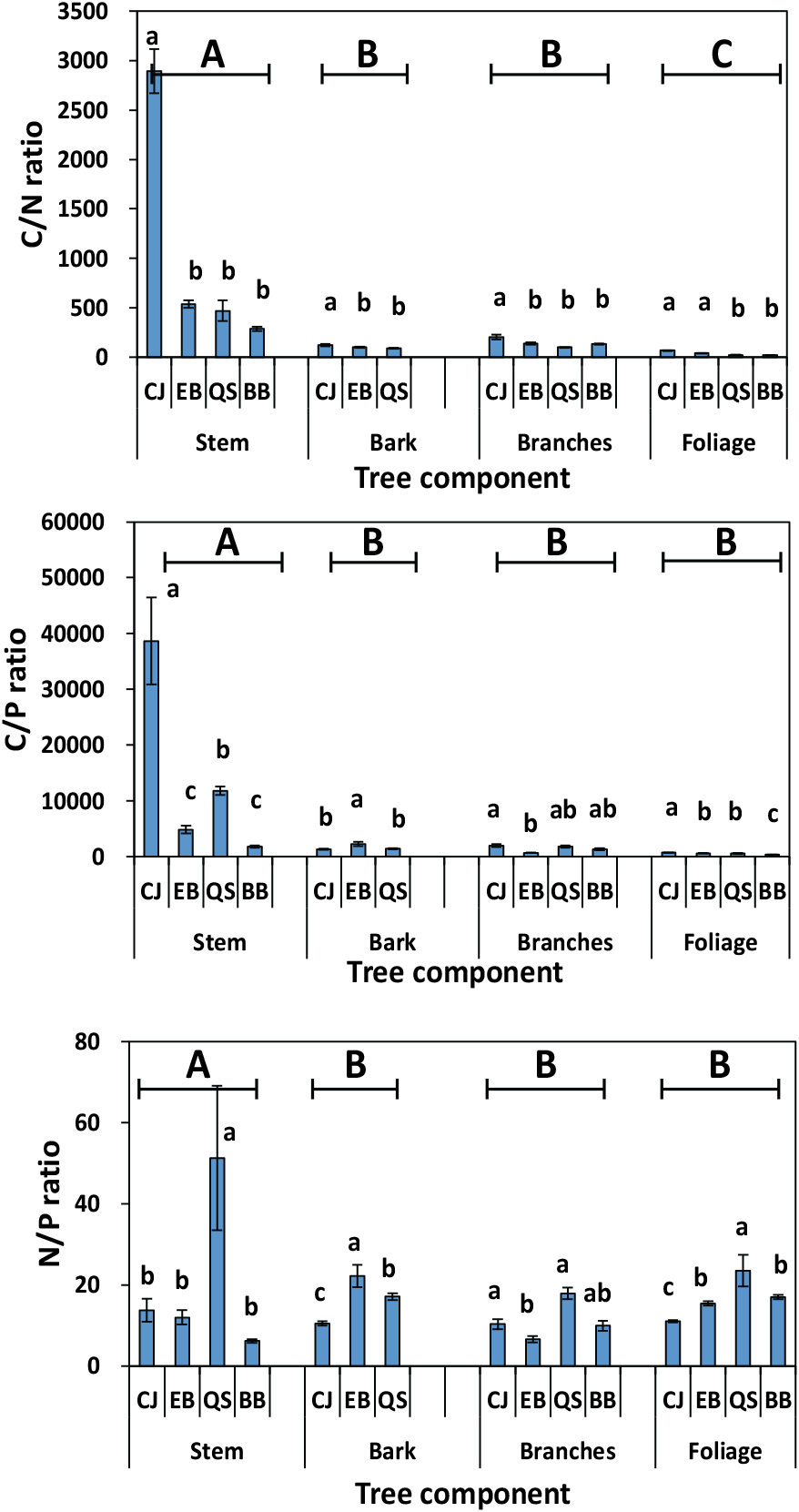
The C:P ratio in stem and foliage was the highest in CJ and the lowest in BB. Generally, the C:P ratio indicates plant P-use efficiency and responses to C fixation and P assimilation. Li et al. (2017) reported that the C:P ratio was positively correlated with plant P-use efficiency but negatively correlated with plant growth rate. This result suggests that BB has high growth rates with a low C:P ratio.
Foliage N:P ratio can determine the potential N and P limitations of plant (Schreeg et al., 2014). A ratio < 14 indicates N limitation, and a ratio >16 indicates P limitation (Mo et al., 2015; Li et al., 2017). The highest N:P ratio in the foliage of QS indicated P limitation in this forest. In addition, the stems or branches in QS showed higher N:P ratios than in EB. Ranges in the N:P ratio except for QS were 6.2-13.7 for stem, 10.5-22.2 for bark, 6.6-10.3 for branches, and 11.0-16.9 for foliage.
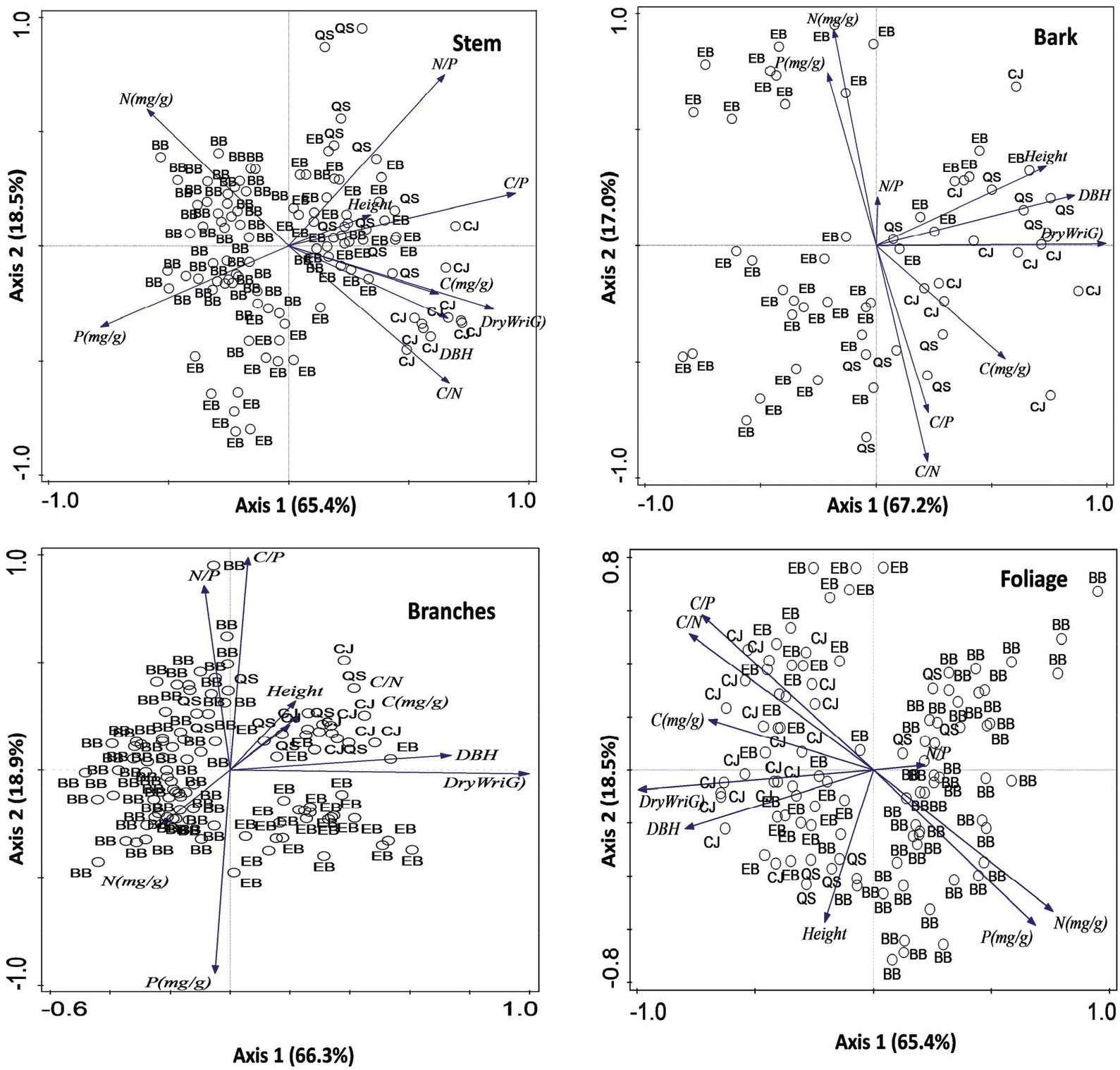
The first principal components (Axis 1) and the second principal components (Axis 2) accounted for 83.9% of the total variation on stem, 84.2% of the total variation on bark, and 85.2% of the total variation on branches, and 83.9% of total variation on foliage, respectively (Figure 3). The stem showed a strong positive correlation between the C concentration and dry weight, but there was a negative correlation between N and dry weight, indicating a dilution effect. The N and P concentrations of foliage and bark were strongly correlated, whereas the concentration of stem and branches was poorly correlated. This result suggests that the correlation of N and P concentrations of plant organs showed different patterns related to organ functions. For example, foliages and phloem within the bark are metabolically active organs, whereas the low nutrient concentrations of stem and branches can be due to the dilution effect caused by biomass. There were positive correlations between C:N and C:P in bark and foliage, whereas C:N and C:P ratios in all plant organs were negatively correlated with N and P concentrations.
Conclusion
Nutrient concentration and stoichiometric patterns in different plant organs responded differently to forests. Due to fast growth, the metabolism in BB requires more N and P, resulting in N and P enrichment in the plant organs. However, the lowest N and P concentrations of foliage and stem in CJ could be associated with the intrinsic genetic factors of coniferous trees. Across all plant organs, the C:N and C:P ratios in the storage organs (stem or branches) were generally higher than those in the metabolic organs (foliage). There were intraspecific differences in the requirements and utilization strategies of different nutrients in the various plant organs of warm-temperate forests at the regional scale studies. These results can be helpful for understanding nutrient cycling patterns by plant organs in warm-temperate forests. In addition, research on the stoichiometric characteristics of forest floor or soil layers can be needed to understand the growth environments in warm-temperate forests.
